Lateral medullary infarction in a patient with Moyamoya disease associated with RNF213 variants: a case report
Article information
Abstract
Background
Moyamoya disease (MMD) is a rare cerebrovascular disease radiologically characterized by progressive bilateral occlusion of the distal portion of the internal carotid artery and compensating collaterals. Herein, we report a case of medullary infarction in a patient with MMD.
Case Report
We present the case of a 54-year-old male with hypertension, hyperlipidemia, and unstable angina with sudden onset dysarthria and ataxia. Diffusion-weighted and T2-weighted images of magnetic resonance imaging showed a high-signal intensity lesion on the right lateral medulla, suggestive of acute infarction. Transfemoral cerebral angiography also demonstrated bilateral middle cerebral artery (MCA) occlusion. Testing of the ring finger protein 213 (RNF213) gene revealed a homozygous p.R4810K variant that was possibly associated with posterior circulation involvement.
Conclusion
When the MCA is occluded in MMD, there is a possibility that medullary infarction may occur due to the mechanism of increased hemodynamic stress on the anastomotic posterior vessels.
INTRODUCTION
Moyamoya disease (MMD) is a rare cerebrovascular disease that is radiologically and clinically characterized by slowly progressive occlusion of the supraclinoid internal carotid artery (ICA) and the circle of Willis, with the simultaneous appearance of natural intracranial and extracranial collaterals [1]. Initial presentation of MMD is generally caused by cerebrovascular events such as cerebral infarction, hemorrhage, transient ischemic attack, epileptic seizures, and sometimes cognitive impairment [2].
While typical symptoms are primarily anterior circulation-related problems, the prevalence of posterior cerebral artery (PCA) involvement has been reported to range from 21.2% to 43.4% in adults and tends to be positively correlated with the ipsilateral ICA stage [3,4]. However, cases of brainstem involvement in MMD have rarely been reported. Here, we report a case of lateral medullary infarction with MMD and multiple vascular risk factors.
CASE REPORT
A 54-year-old right-handed male visited the emergency room with the sudden onset of dysarthria and ataxia. He had hypertension, hyperlipidemia, and a history of coronary angioplasty and stent insertion for unstable angina. Because of these underlying diseases, aspirin, clopidogrel, rosuvastatin, ramipril, trimetazidine, carvedilol, and nifedipine were prescribed.
Neurological examination revealed dysarthria and truncal ataxia. He also experienced pain and thermal sensory impairment in the left limbs, trunk, and right side of the face. The initial National Institutes of Health Stroke Scale score was 4. His vital signs were stable, with high blood pressure (blood pressure 167/92 mm Hg, pulse rate 75 beats/min, body temperature 36.8 °C, and respiratory rate 16/min). Electrocardiography revealed a normal sinus rhythm, and chest radiography findings were normal. The fasting blood glucose level was 105 mg/dL, and the HbA1c level was 6.0%. Additional laboratory tests revealed normal cholesterol (155 mg/dL), total triglyceride (127 mg/dL), and low-density lipoprotein cholesterol (95 mg/dL) levels. Laboratory test results for hypercoagulation causes were negative. Other serological parameters, including erythrocyte sedimentation rate and C-reactive protein levels, were within normal limits.
Diffusion-weighted and T2-weighted magnetic resonance imaging (MRI) revealed a high-signal intensity lesion in the right lateral portion of the medulla, suggestive of acute infarction (Fig. 1). Transfemoral cerebral angiography (TFCA) showed bilateral M1 occlusion, and distal middle cerebral artery (MCA) flow was supplied by the collaterals (Fig. 2). The TFCA also showed that the vertebral artery supplied a portion of the right temporal and left temporo-occipital regions (Fig. 3). Transcranial Doppler (TCD) showed increased blood flow velocities in both the anterior cerebral arteries (ACA; 108.8 cm/sec in the left ACA and 93.3 cm/sec in the right ACA) and the right MCA (121.4 cm/sec). MMD was suggested, and subsequent genetic testing for ring finger protein 213 (RNF213) revealed a homozygous p.R4810K variant.

Magnetic resonance imaging of the brain. (A, B) Diffusion-weighted imaging showed high-signal intensities in the right lateral medulla. (C, D) Apparent diffusion coefficient imaging showed low signal intensities in the corresponding area.
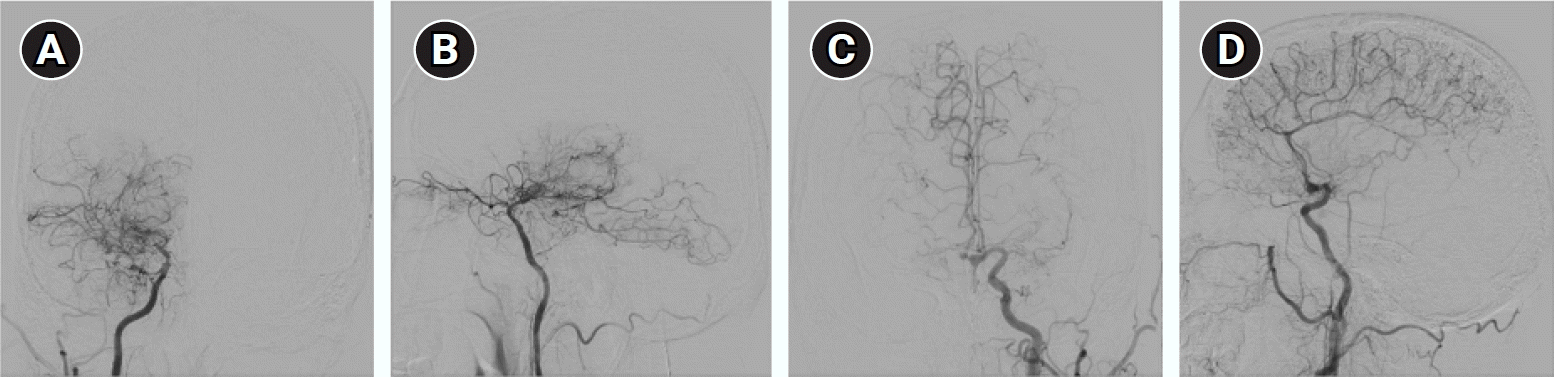
(A-D) Transfemoral cerebral angiography revealed bilateral middle cerebral artery (MCA) occlusion and distal MCA flow was supplied by collaterals.
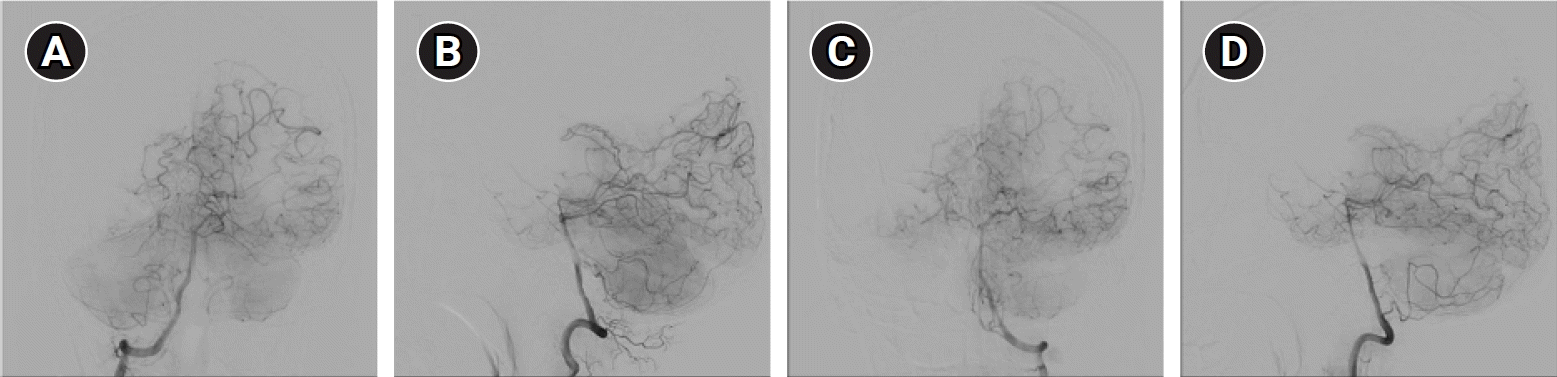
(A-D) Transfemoral cerebral angiography revealed vertebral artery supplied some portion of right temporal and left temporo-occipital regions.
The patient was prescribed cilostazol, clopidogrel, and 40 mg of atorvastatin. At discharge, a maintenance regimen of dual antiplatelet therapy and atorvastatin was prescribed, which was administered upon admission. One month after symptom onset, his condition remained stable except for mild residual dysarthria.
DISCUSSION
As the distinction and diagnostic criteria between MMD and moyamoya syndrome have been revised, radiological diagnostic criteria have been emphasized [5]. TFCA should demonstrate arterial stenosis or occlusion centered at the distal ends of the bilateral or unilateral intracranial carotid arteries, arterial phase occlusion, or moyamoya vessels near the stenotic lesion, suggesting an abnormal vascular network.
Several monogenic moyamoya syndromes present with the radiological features of MMD and are associated with several pathways involved in the development of moyamoya angiopathy [6]. Among several MMD susceptibility genes, RNF213 14429G→A (rs112735431; resulting in p.R4810K) was identified as a major susceptibility gene for MMD and a major founder variant in East Asian countries, especially in Japan and South Korea [7]. The RNF213 p.R4810K variant is not common in the general Korean population, and these genetic polymorphisms are present in only 0.5% to 2% of the general East Asian population [7,8].
RNF213 may play an important role in the progressive stages of MMD as well as in vascular pathology, such as angiogenesis and collateral vessel development in PCA involvement [9,10]. Furthermore, a study on Korean subjects showed that patients with RNF213 mutations had fewer collateral flow patterns from the PCA to the anterior circulation and more PCA involvement than patients with MMD without RNF213 mutations [9].
MMD-associated lateral medullary infarction and/or vertebral artery occlusion have rarely been reported in the past, and its mechanisms remain unclear [11,12]. As mentioned above, the involvement of the posterior circulation in MMD is relatively rare, and even when involved, it is usually present in PCA. Additionally, although symptoms occur because of the vertebrobasilar artery, most cases are caused by hemorrhage or total vertebrobasilar occlusion, which is different from the present case, which is believed to be caused by hemodynamic stress. From an embryological perspective, because the distal part of the PCA originates from the primitive ICA, most MMD associated with the posterior circulation are considered to be of the primitive ICA system-related type, the primitive vertebrobasilar artery system [13].
Although genetic testing for RNF213 in our patient showed a homozygous p.R4810K variant, he also had multiple vascular risk factors such as hypertension, hyperlipidemia, and smoking. This case did not meet the 2021 criteria for MMD because there was no distal ICA stenosis or occlusion, despite TFCA demonstrating bilateral MCA occlusion. Additionally, atherosclerotic changes related to traditional risk factors may cause lateral medullary infarctions. However, the small, abnormal, and reticular collateral vessels typically observed in MMD, including the posterior collaterals, were observed at multiple sites. TCD also demonstrated increased flow velocity in both the ACA and MCA, consistent with MMD. Furthermore, since the RNF 213 mutation appeared in this case, it is reasonable to consider the possibility that MMD is progressing.
The possibility of occlusion of the MCA, which may increase the hemodynamic stress on the anastomotic posterior vessels due to MMD, may also be considered the cause in this case. Although MMD requires accurate recognition and application of the diagnostic criteria and clinical features, it is necessary to suspect and consider MMD because of its potential to cause various symptoms.
Notes
Ethics statement
This case was reviewed and approved by the Ethics Committee of CHA Bundang Medical Center (No. CHAMC IRB 2023-11-019). The requirement of informed consent was waived. The CARE guidelines were followed to enhance the quality and standardization of the reported cases.
Conflict of interest
No potential conflict of interest relevant to this article.
Author contributions
Conceptualization: YJR, HSK. Methodology: all authors. Formal analysis: all authors. Data curation: YJR, HSK. Visualization: all authors. Project administration: YJR, HSK. Writing–original draft: YJR. Writing–review & editing: all authors.
