Effects of sufficient anticoagulation on ischemic stroke outcomes in patients with nonvalvular atrial fibrillation
Article information
Abstract
Background
Optimal anticoagulation therapy reduces the risk of ischemic stroke in patients with nonvalvular atrial fibrillation (AF). Therefore, we aimed to evaluate the effects of prior anticoagulation therapy with vitamin K antagonists (VKAs) and direct oral anticoagulants (DOACs) on ischemic stroke outcomes in patients with nonvalvular AF.
Methods
We enrolled 487 patients with ischemic stroke and nonvalvular AF between January 2013 and August 2020. The infarct volume was semi-automatically evaluated using diffusion-weighted magnetic resonance imaging. Patients were categorized into no anticoagulation, undertreated anticoagulation, and sufficient anticoagulation (with VKA or DOAC) groups based on their pre-admission anticoagulant use, and the clinical characteristics were compared between the groups.
Results
Among the included patients, 374 (76.8%), 50 (10.3%), 10 (2.1%), and 53 (10.9%) patients received no anticoagulants, were undertreated with a VKA, were sufficiently treated with a VKA, and received DOACs, respectively, before stroke. Multivariate analysis revealed that optimal anticoagulation was independently associated with a low risk of severe stroke (odds ratio, 0.553; 95% confidence interval, 0.308–0.992; P=0.047). Additionally, the DOAC group had a significantly smaller mean infarct volume than the other groups (45.8±73.2, 45.0±69.1, 30.9±24.7, and 12.6±24.9 mL in the no anticoagulation, insufficient VKA, sufficient VKA, and DOAC groups, respectively; P=0.011).
Conclusion
Sufficient pre-stroke anticoagulation is associated with mild stroke severity and good outcomes at 3 months post-stroke. Additionally, pre-stroke DOAC treatment is associated with smaller infarct volume in patients with ischemic stroke and nonvalvular AF.
INTRODUCTION
Atrial fibrillation (AF) is an important risk factor for ischemic stroke, and 10%–15% of all ischemic strokes occur in patients with AF [1]. Previous studies have demonstrated that optimal anticoagulation using vitamin K antagonists (VKAs), warfarin, and direct oral anticoagulants (DOACs) reduces the risk of ischemic stroke in patients with AF [2-7]. Therefore, international guidelines recommend sufficient anticoagulation with VKAs and DOACs for primary and secondary prevention, respectively, of cardioembolic ischemic stroke in patients with nonvalvular AF [8,9]. Moreover, compared to no anticoagulation therapy, sufficient VKA and DOAC therapy reportedly reduced ischemic stroke severity and improved clinical outcomes in patients with AF [10-14]. Furthermore, previous studies reported that DOAC therapy reduced the initial severity of ischemic stroke similar to sufficient VKA treatment [15,16]. However, the clinical impact of optimal anticoagulation with different treatment modalities, such as VKAs and DOACs, on initial stroke severity, infarct volume, and short-term outcomes in patients with acute cardioembolic ischemic stroke and nonvalvular AF remains unknown. Therefore, we aimed to investigate the association of pre-stroke anticoagulation therapy with VKAs and DOACs as well as stroke severity and outcomes in patients with ischemic stroke and non-valvular AF.
METHODS
Study population
We retrospectively included patients who were admitted to our hospital with acute ischemic stroke within 7 days of symptom onset, between January 2013 and August 2020. Of the 603 patients with cardioembolic ischemic stroke and AF who were initially enrolled, those with valvular AF (n=69) and those who did not undergo diffusion-weighted imaging (DWI) during hospitalization after ischemic stroke (n=37) were excluded. Finally, 487 patients with ischemic stroke and nonvalvular AF were included.
Baseline characteristics and clinical information
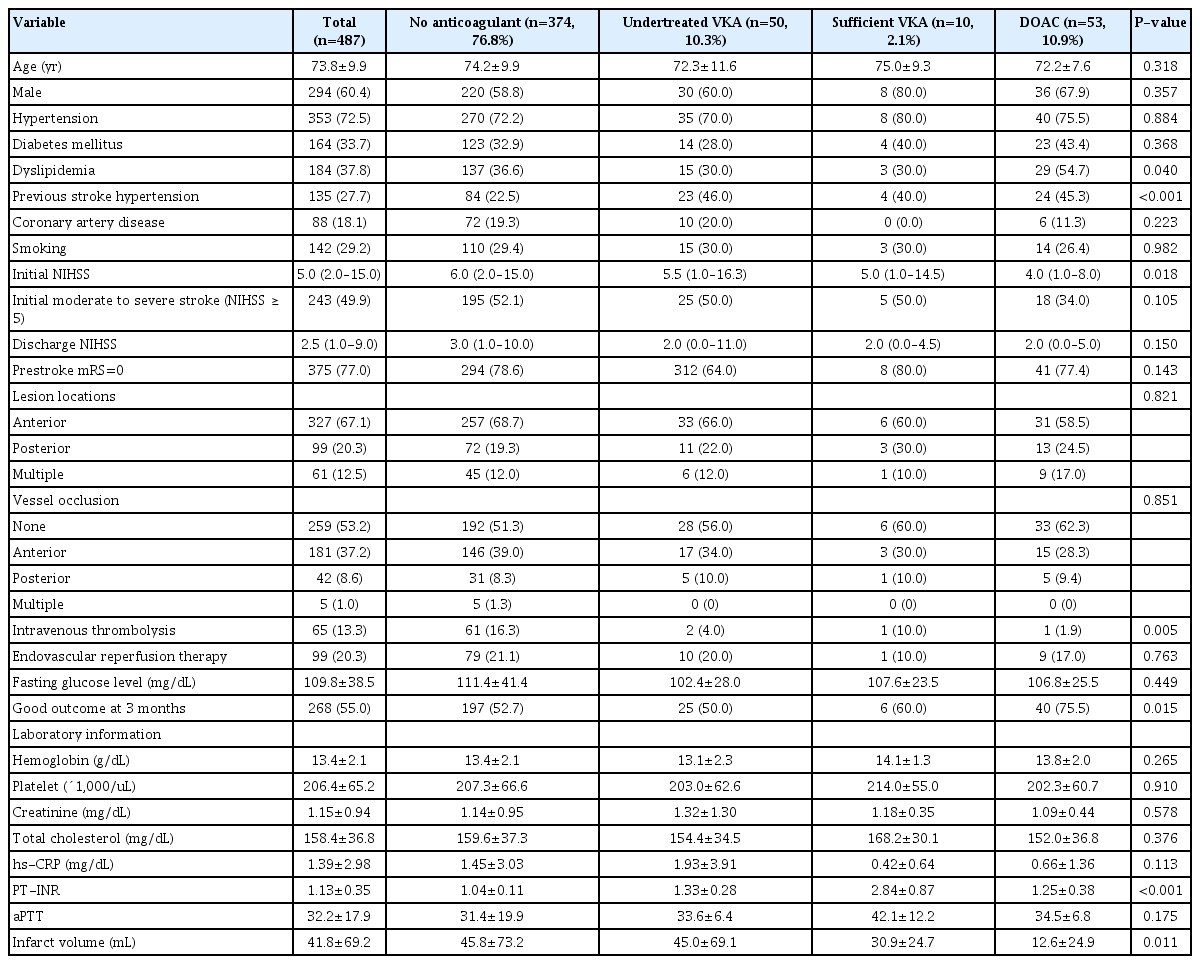
Clinical data regarding patient demographics, vascular risk factors, location of vessel occlusion, lesion location, reperfusion therapy, medication history, and laboratory findings were retrieved from the electronic medical records. All patients underwent laboratory examinations, including glucose and lipid profiling, blood cell counts, high-sensitivity C-reactive protein analysis, prothrombin time-international normalized ratio (PT-INR), and activated partial thromboplastin time, at admission. For the purpose of this study, pre-admission anticoagulation treatments were categorized into four groups: (1) no anticoagulation therapy, (2) undertreated anticoagulation (subtherapeutic dose of VKA with a PT-INR of <2 at admission), (3) sufficient VKA therapy (therapeutic dose of VKA with a PT-INR of ≥2 at admission) [8,9], or those on DOACs. Sufficient anticoagulation therapy was classified as sufficient VKA and sufficient DOAC. The National Institutes of Health Stroke Scale (NIHSS) score, which was the primary outcome at discharge, was used to measure stroke severity at admission, which was the primary outcome, and at discharge. Patients with an NIHSS score of ≥5 were classified as having moderate to severe stroke [17]. The functional outcome at 3 months post-stroke, which was the secondary outcome, was evaluated using the modified Rankin Scale (mRS). Patients were assigned to either the “good outcome” (mRS score ≤2) or “poor outcome” (mRS score ≥3) group. Additionally, infarct volume was analyzed according to the pre-admission anticoagulation therapy received by the patients.
Radiological assessment
Brain magnetic resonance (MR) images were obtained using a 1.5 T (Signa HDxt, GE Healthcare; n=114) or 3.0 T (Verio, Siemens [n=91]; Discovery 750, GE Healthcare [n=158]; Magnetom Skyra, Siemens [n=51]; Ingenia CX, Philips [n=73]) machine. Moreover, we extensively acquired MR images using DWI (repetition time/echo time: 6,000–10,000/65–80 for 3.0 T and 5,000–9,000/65–75 for 1.5 T) [18-20]. The lesion locations were classified as anterior circulation, posterior circulation, or multiple territories. The cerebral infarct volumes of the lesions were analyzed on DWI using Medical Imaging Processing, Analysis, and Visualization (MIPAV, ver. 10.0.0; National Institutes of Health) [21], by an investigator blinded to the clinical information. Large-vessel occlusion was evaluated using brain computed tomography angiography or MR angiography.
Statistical analysis
Baseline characteristics are presented as frequencies (percentages). Continuous variables with normal distributions are presented as means±standard deviations, whereas variables with non-normal distributions are presented as medians (interquartile ranges). In the univariate analysis, the proportions of categorical variables were compared using Pearson chi-square test or Fisher’s exact test, as appropriate. The relationships between continuous variables and anticoagulation therapies were analyzed using the Kruskal-Wallis test or one-way analysis of variance. Associations between outcomes and the levels of anticoagulation therapy were analyzed using logistic regression analysis. Covariates with a significance level of P<0.05 in the univariate analyses or clinically important variables were used for adjusting the multivariate analysis. Statistical significance was set at P<0.05. All statistical analyses were performed using IBM SPSS ver. 25.0 (IBM Corp.) and GraphPad Prism ver. 9 (GraphPad Software).
RESULTS
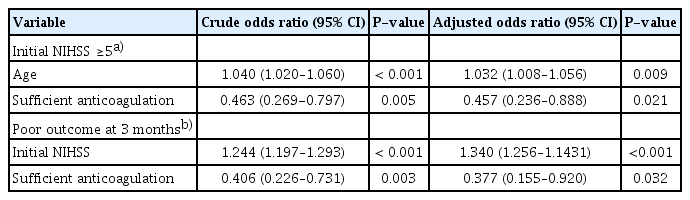
Of the 487 patients included in this study, 374 (76.8%) received no anticoagulant medication, 50 (10.3%) were undertreated with VKA, and 63 (12.9%) received sufficient anticoagulation (10 [2.1%] received VKAs and 53 [10.9%] received DOACs). Table 1 presents the clinical characteristics of the included patients. Their mean age was 73.8±9.9 years, and 294 (60.4%) of them were men. There were significantly more patients with a history of stroke/transient ischemic attack and dyslipidemia in the undertreated and sufficient anticoagulation groups than that in the no anticoagulation group (P<0.001). Patients who received pre-stroke anticoagulation therapy were less likely to undergo intravenous thrombolysis upon admission (P=0.002). There were no significant differences in lesion location or vessel occlusion among the four groups (Table 1). The sufficient anticoagulation group had a lower initial NIHSS score, better outcomes at 3 months post-stroke, and lower infarct volume than the no anticoagulation and undertreated VKA groups (no anticoagulation vs. undertreated VKA vs. sufficient anticoagulation: NIHSS score: 6.0 [2.0–15.0] vs. 5.5 [1.0–16.3] vs. 4.0 [1.0–9.0], P=0.008; good outcomes at 3 months: 52.7% vs. 50.0% vs. 73.0%, P=0.008; infarct volume: 45.8±73.2 vs. 45.0±69.1 vs. 15.5±25.6 mL, P=0.005 (Table 1). Additionally, the DOAC group had a lower initial NIHSS score (P=0.018) and better outcomes at 3 months post-stroke (P=0.015) than the VKA group (Table 2). Furthermore, the DOAC group had the lowest infarct volume among the four treatment groups (no anticoagulation vs. undertreated VKA vs. sufficient VKA vs. DOAC: 45.8±73.2 mL vs. 45.0±69.1 mL vs. 30.9±24.7 mL vs. 12.6±24.9 mL, P=0.011) (Table 2, Fig. 1). However, sufficient anticoagulation was negatively associated with initial stroke severity (NIHSS score ≥5) and poor outcome in univariate analysis (Table 3), and was independently negatively correlated with initial moderate to severe stroke (odds ratio [OR], 0.467; 95% confidence interval [CI], 0.236–0.888; P=0.021) and poor outcome at 3 months post-stroke (OR, 0.377; 95% CI, 0.155–0.920; P=0.032) (Table 3) in multivariate analysis.
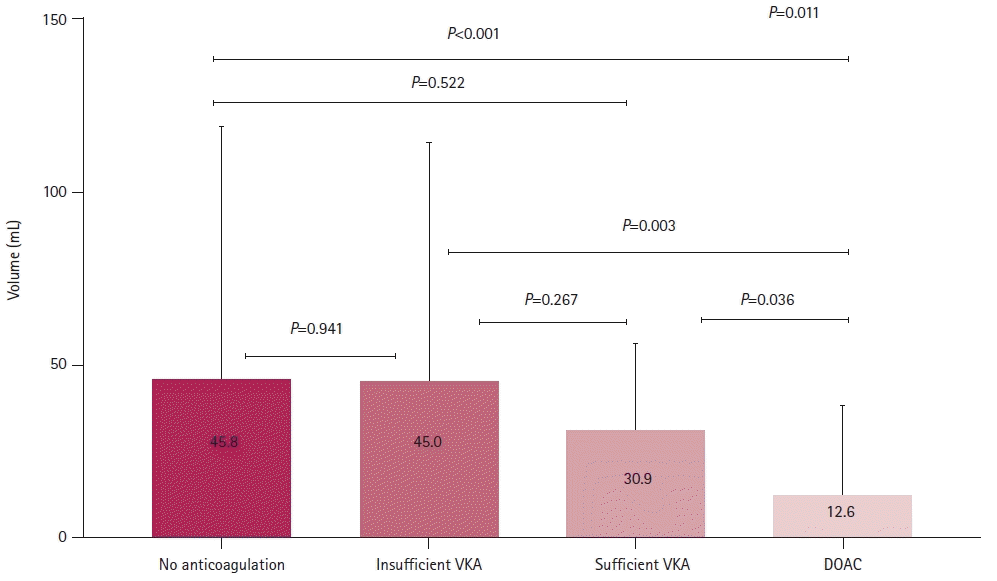
Comparison of infarct volume according to pre-stroke anticoagulation. The sufficient anticoagulation group (sufficient vitamin K antagonist [VKA] and direct oral anticoagulants [DOACs]) had a lower infarct volume than no anticoagulation and undertreated VKA groups. Furthermore, the DOAC group had the lowest infarct volume by a significant margin among the four treatment groups.
DISCUSSION
This study showed that compared to no or undertreated anticoagulation, sufficient pre-stroke anticoagulation with VKA and DOAC was associated with milder stroke and was independently related to a better outcome at 3 months post-stroke in patients with nonvalvular AF. Additionally, the DOAC group had the lowest initial NIHSS score and smallest infarct volume among the four anticoagulation therapy groups.
Patients with AF who receive sufficient pre-stroke VKA therapy have a lower risk and severity of stroke, and better outcomes than those who do not receive anticoagulation [10-14]. Moreover, DOAC therapy was more effective than other anticoagulant therapies in reducing infarct volume, arterial occlusion, and stroke severity in patients with AF who experienced anterior circulation ischemic stroke [15,16]. Although previous studies have reported the occurrence of major artery occlusion leading to ischemic stroke in patients with AF and negative associations between VKA or DOAC therapy and initial stroke volume, few studies have evaluated the relationship between sufficient anticoagulation and initial stroke severity, functional outcome, and infarct volume according to the type of pre-stroke anticoagulation therapy in patients with AF and ischemic stroke in different vascular territories. In our study, sufficient pre-stroke anticoagulation reduced stroke severity and infarct volume, and improved functional outcomes in patients with nonvalvular AF, regardless of the vascular territory involved. Moreover, DOAC therapy resulted in decreased stroke severity and improved functional outcomes, consistent with the findings of previous studies [15,16].
Sufficient anticoagulation therapy leads to a decrease in the size of thrombi and emboli, thereby preventing large artery occlusion and decreasing the infarct volume, resulting in mild initial symptoms of acute ischemic stroke [13,22,23]. Small thrombi, which occur in patients consistently receiving sufficient anticoagulation, are indirectly related to the ultra-early recanalization of vulnerable thrombi, thereby preventing large artery occlusion. Owing to minimal fluctuations in its anticoagulation effect, DOAC therapy consistently provides optimal anticoagulation therapy compared to VKA therapy. Therefore, DOAC therapy is at least as effective as VKA therapy in preventing stroke occurrence and reducing initial stroke severity [15,16]. Consequently, the recommended anticoagulation strategy for the secondary prevention of ischemic stroke in patients with AF has been changed from VKA to DOAC, and additional indications for DOAC use have been suggested [4-9]. Sufficient anticoagulation can be attributed to the inhibition of the thrombotic system through the acceleration of thrombolysis, which may lead to gradual fibrinolysis in the thrombi. Therefore, therapeutic anticoagulation can reduce infarct size by preventing microvascular thrombosis after ischemic stroke [24], which may explain the lower stroke severity and better outcomes in the DOAC group than that in the VKA group.
In our study, stroke severity, infarct volume, and outcomes of the insufficient anticoagulation group were similar to those of the no anticoagulation group. In contrast, subtherapeutic anticoagulation with VKA was associated with poor medication adherence. A PT-INR in the subtherapeutic range in patients receiving VKAs may lead to a transient hypercoagulable state due to the difference in plasma half-life between procoagulation factors II and X and anticoagulant proteins C and S [25,26]. Therefore, it is pharmacokinetically possible that, compared to the no anticoagulation group, the subtherapeutic group had similarly sized or larger thrombi, which may have resulted in an increased infarct volume [27]. Hence, well-managed oral anticoagulant treatment may reduce stroke severity and improve functional outcomes of ischemic stroke in patients with nonvalvular AF.
However, this study had some limitations. First, this was a retrospective study; therefore, there is a possibility of selection bias and uncontrolled factors in the clinical scenario. Second, pre-stroke anticoagulant status was defined based on prescription information, whereas drug adherence data for DOACs were not systematically collected. Third, we did not measure the DOAC plasma levels using calibrated Xa activity or hemoclot assays, which may have affected the findings in the DOAC treatment group. Finally, we did not differentiate between paroxysmal, persistent, or permanent AF. However, all AF types are high-risk factors for embolic vascular events.
In conclusion, sufficient anticoagulation therapy is associated with decreased stroke severity and infarct volume, and improved functional outcomes. Patients with valvular AF who received DOAC treatment prior to ischemic stroke onset showed small infarct volumes and good functional outcomes at 3 months post-stroke. Therefore, sufficient anticoagulation with VKAs and DOACs could improve ischemic stroke outcomes in patients with nonvalvular AF. However, further large-scale clinical studies are required to determine what constitutes sufficient anticoagulation and to evaluate stroke severity and outcomes after ischemic stroke caused by nonvalvular AF.
Notes
Ethics statement
This study was approved by the Institutional Review Board (IRB) of the Seoul National University Hospital (No. H-1009-062-332). The need for informed consent was waived by the board because of the retrospective nature of the study. All the procedures were performed in accordance with the relevant guidelines and regulations of the IRB of the Seoul National University Hospital.
Conflict of interest
Tae Jung Kim is an editor-in-chief, and Soo-Hyun Park and Sang-Bae Ko are editorial board members of the journal, but they were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: TJK, SBK. Methodology: TJK, SBK. Software: TJK, SHP. Validation: YK, SHP. Formal analysis: TJK. Investigation: TJK, YK, SHP. Resources: TJK, SBK. Data curation: TJK. Visualization: TJK. Supervision: SBK. Project administration: TJK, SBK. Writing–original draft: TJK. Writing–review & editing: TJK, SBK.